Stay up-to-date with the latest news and resources from P&F Products & Features GmbH. As a leading heart valve manufacturer, we are committed to providing healthcare professionals with the knowledge and tools they need to deliver the best possible patient outcomes. Explore our resources, including the latest product innovations, in-depth case studies, and press releases covering recent company developments in the field of cardiology.
Publications

Patient Selection for Transcatheter Tricuspid Intervention: Timing Is Everything
A recent review published in the Structural Heart Journal reinforces an important reality that P&F Products & Features—and many Heart Teams worldwide—see every day: patients with severe tricuspid regurgitation are still being

P&F Hosts Transformative 2025 Sales Meeting in Brazil and LATAM
A Milestone in Innovation and Collaboration From March 24 to 26, 2025, P&F Products & Features proudly hosted the 2025 Sales Meeting for Brazil and LATAM in São José do Rio Preto.
Brazil Celebrates its 100th Implant of the TricValve System
Celebrating the 100th implantation of the Tricvalve system in Brazil! We are thrilled to announce the 100th transcatheter caval valve implant using TricValve’s innovative technology for patients with severe tricuspid regurgitation in
PRESS RELEASEs

P&F Secures EU MDR CE Certification for TricValve® System
P&F secures CE Mark Certification under the EU Medical Device Regulation (MDR) 2017/745 regulations for TricValve® Bicaval Valve System, A Breakthrough Minimally Invasive Therapy for Severe Tricuspid Regurgitation Vienna, Austria, Feb. 5,

P&F secures FDA approval for TRICAV II pivotal clinical trial for severe tricuspid regurgitation
TRICAV II will evaluate the safety and effectiveness of the TricValve® Transcatheter Bicaval Valve System Wilmington, Delaware, Dec. 12, 2025 — P&F USA, Inc., the U.S. subsidiary of global heart valve manufacturer

P&F Highlights Global Progress in Tricuspid Valve Therapies at TCT 2025
San Francisco, CA — October 2025 The global forum for interventional cardiology, Transcatheter Cardiovascular Therapeutics (TCT) conference, once again brought together the world’s leading experts in interventional cardiovascular medicine to explore the

P&F Products & Features GmbH wins esteemed Houska Prize for innovation and excellence
P&F Products & Features GmbH is proud to announce that it has been awarded the esteemed Houska Prize for outstanding innovation and excellence in the “Research and Development in Small and Medium-Sized

P&F Products and Features GmbH Appoints Dr. Roswitha Wolfram as Global Chief Medical Officer
Long-time healthcare executive to lead worldwide medical team P&F Products and Features GmbH (P&F), a leading global medical technology company focused on developing innovative minimally invasive cardiology solutions, today announced the appointment

TricValve is Shortlisted for the Prestigious HOUSKA PRIZE 2025
P&F Products & Features, GmbH, Austria, is honored to be among the 15 short-listed finalists for the esteemed Houska Prize 2025 — Austria’s largest privately awarded prize fund for application-driven research. TricValve® – Transcatheter Bicaval Valve
Vienna, February 24th 2022
P&F Products & Features goes U.S. – first implantation of the TricValve Transcatheter Bicaval Valves System
Vienna, May 18th 2021
P&F Products & Features GmbH gains CE Mark approval for TricValve® Transcatheter Bicaval Valves System
SINGAPORE, October 2021
THE STRAITS TIMES: More minimally invasive treatment options available in S´pore for patients with leaky heart valve
VIENNA, December 22nd 2020
TricValve® Transcatheter Bicaval Valves System granted Designation as Breakthrough Device by the U.S. Food and Drug Administration (FDA)
Short Cases
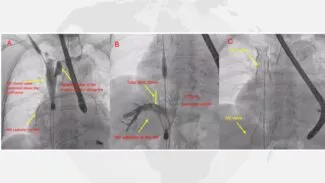
First TricValve® Case in the Philippines
First TricValve® Case in the Philippines Demonstrates Promising Outcomes for Severe Tricuspid Regurgitation A recent case published in the Philippine Journal of Cardiology documents the country’s first successful implantation of the TricValve®

Bicaval TricValve Tricuspid Regurgitation: Late-Breaking PCR London Valves 2024
Source Data presented at PCR London Valves 2024 showed that caval valve implantation (CAVI) with the TricValve® system demonstrates an acceptable safety profile and significant clinical benefits in highly comorbid patients with severe

TricValve® Case: Salamanca
Performed in Spain at the University Hospital of Salamanca Dr. Javier Martín Moreiras MD, PhD Interventional Cardiologist and Associated Professor of Cardiology. University Hospital of Salamanca, Spain An 83-year-old woman with a
